CGLytics is Glass Lewis’ exclusive global partner for their P4P Methodology from January 1, 2020
Read More
CGLytics is now the sole provider of compensation data and analytics tools to Glass Lewis, informing their research and recommendations for Say on Pay proposals. CGLytics is the only distributor in the market that provides access to Glass Lewis’ compensation tools, including Glass Lewis’ P4P Methodology, Equity Compensation Model and their new, proprietary 2020 peer group methodology.

Latest Industry News, Views & Information
- All
- Activism
- AI
- Board Effectiveness
- Board Skills
- CEO
- CEO pay
- Corporate Governance
- Diversity
- Equity Compensation
- Executive Pay
- Glass Lewis
- Governance Analytics
- Governance Risk
- Investment
- Investor Activism
- Shareholders
- SRD II
- Succession Planning
Understanding ESG & Annual Incentive Plan
Understanding ESG & Annual Incentive Plan ESG refers to a series of environmental, social and governance criteria taken into consideration by the funds during the investing process. Investing in ESG funds allows shareholders to support companies in transition, that wish to act and develop in a more sustainable and responsible manner. In practice, many indicators … Continue reading “Understanding ESG & Annual Incentive Plan”
Pay for Performance: The Largest Institutional Investors’ View
Pay for Performance: The Largest Institutional Investors’ View Executive compensation has been one of the trickiest issues within the corporate governance space as of late. Across the board, there seems to be no end in sight to finding the perfect compensation package or philosophy for corporate executives. In this article, we will discuss the … Continue reading “Pay for Performance: The Largest Institutional Investors’ View”
How to design your peer group for compensation benchmarking
How to design your peer group for compensation benchmarking Given the scrutiny on executive compensation in recent years, it is critical to make sure that your company’s executive pay reflects its performance and aligns with the market. Therefore, it is essential for companies to have an appropriate peer group for performance benchmarking, compensation program … Continue reading "How to design your peer group for compensation benchmarking"
5 Long Term Incentive Plans Overwhelmingly Voted by Investors
5 Long Term Incentive Plans Overwhelmingly Voted by Investors Executive’s remuneration consists of fixed and variable elements. The fixed element component of Executive compensation consists of the base salary and pension of the executives. This element of pay is usually independent of performance, meaning that they will be paid regardless of the executive’s performance. Due … Continue reading "5 Long Term Incentive Plans Overwhelmingly Voted by Investors"
How to Design your Annual Incentive Plan During a Pandemic
How to design your annual incentive plan during a pandemic The novel COVID-19 pandemic has impacted many areas of the current landscape, including the socio-economic landscape and macroeconomic environment. Initially it was difficult to predict how long the pandemic was likely to last, however it has certainly continued longer than initial indications led us … Continue reading "How to Design your Annual Incentive Plan During a Pandemic"
How the SEC’s new proxy voting rules will impact executive compensation
There are many software applications and tools now available to support compensation decisions, but what should be taken into consideration before purchasing? This 5-minute guide details what Compensation Committees, Heads of Reward and Compensation Professionals should take into account when selecting software and tools for Say-on-Pay decisions.
Activist Investor Focus in 2020
View the facts and figures of activist investor campaigns in 2020. What have been the key target areas and how can companies be better prepared?
S&P 500 Banking Industry’s Response to COVID-19
CGLytics examines how S&P 500 banks responded to the volatility of the pandemic prior to the Fed’s announcement to cap bank dividends and prohibit share repurchases until Q4 following its annual stress test of banks.
How to independently and efficiently benchmark executive compensation for Say-on-Pay
There are many software applications and tools now available to support compensation decisions, but what should be taken into consideration before purchasing? This 5-minute guide details what Compensation Committees, Heads of Reward and Compensation Professionals should take into account when selecting software and tools for Say-on-Pay decisions.
CNBC Report: More activist investors to focus on corporate governance and executive pay
This week CGLytics CEO discussed the increase in activist investor activity with CNBC Street Signs. New research from CGLytics reveals that activist investors are broadening their focus.
Diversity on the Board? Metrics Used by Fortune 100 Companies
Examining the diversity of Fortune 100 boards and questioning the metrics currently used to disclose diversity. Are Fortune 100 companies providing the complete picture?
COVID-19: Changes to Executive and Shareholder Pay in Europe’s Biggest Banks
To evaluate the financial industry’s response to the COVID-19 crisis, CGLytics reviews the executive compensation changes and dividend amendments of 20 listed banks across Europe.
2020 CEO Pay Review: The Top 50 Highest Paid CEOs
As proxy season progresses and companies file their annual reports, CGLytics surveys the world’s highest paid CEOs (so far) and looks at how executive compensation has grown since the last year.
AGMs: Tactics for a Plague Year
In a time of crisis and confusion, shareholders are more eager than ever to get answers from their boards and management. Yet holding traditional AGMs is nearly impossible. What are the best options for running AGMs during a plague year?
A diverse supervisory board: This is how to unlock a wealth of talent
Aniel Mahabier, CEO of governance data specialist CGLytics, welcomes the fact that selection committees are using corporate governance analytics to assess the diversity of their own supervisory board. Technology is bridging the gap between the available talent and the knowledge and experience that committees already have in-house.
What’s your flavor? Companies get a taste of CEO pay for the proxy season
This article, originally published in Dutch in Mgmt. Scope, CGLytics examines CEO compensation issues going into the 2020 proxy season
CEO Pay Continues to Increase, but Performance Often Lags
Shareholders, including large institutional investors, are continuing the growing momentum to link executive pay to company performance.
Glass Lewis New Peer Group Methodology for Say on Pay
Due to Glass Lewis now using CGLytics data to power their Say on Pay recommendations and adapting their methodology to peer-based approach, what is the impact on companies’ pay for performance gradings?
Gender diversity in Spanish boardrooms
In Spain, the Comision Nacional de Mercado de Valores (CNMV), has put a series of changes to the corporate governance code of public companies under consultation. This is widely regarded as one of the most relevant proposed amendments relating to gender diversity in boardrooms.
Randstad make data-based decisions going into the AGM season
“At Randstad we are looking at the remuneration policy on a continual basis and it’s an important topic. Prior to the AGM we want to fully understand how all our stakeholders look at our remuneration policy. “
Reflection on 2019 Executive Pay: Belgium and Luxembourg
In the recent report published by PwC, using CGLytics data and analytics, the critical trends from the 2019 proxy season for Belgium and Luxembourg listed companies surrounding executive compensation were revealed.
The DOs and DON’Ts when rethinking incentive plans
Why have 75% of first-time say-on-pay votes failed in 2019? A large number of negative votes can be attributed to incentives. Companies need to rethink their incentive plans and make sure metrics truly benchmark performance.
Remuneration policy: Directors reward attracts more and more attention
A well-founded remuneration policy is no longer optional. The new European Shareholder Rights Directive demands transparency around remuneration of directors.
Pressure from stakeholders brings about change
In an increasing number of companies, remuneration based on short-term results is giving way to a remuneration structure based on long-term performance. Companies should be able to indicate how the CEO’s remuneration contributes to long-term value creation, and be prepared to discuss their performance in this area.
SRD II and the ramifications for disclosure obligations
With the proxy season fast approaching SRD II is top of mind. Learn about the implications SRD II will have on disclosure of executive pay and corporate goverannce.
How Glass Lewis improved their executive compensation analysis and Say on Pay recommendations for European markets
Andrew Gebelin from Glass Lewis talks through how he and his team of analysts have benefited from using the CGLytics data and tools to improve their executive compensation analysis and Say on Pay recommendations for European markets.
The increasing trend of shareholder opposition to executive pay
Votes against executive remuneration are growing. In this article we look at this change in the European indices and the S&P500.
Equity Incentive Schemes: Examining the rationale behind shareholder rejection
Two historical examples of organizations that have had their stock option plans rejected by shareholders include Red Lion Hotels and HomeAway. How could they have reduced the likelihood of rejected plans? Read to find out
The increasing popularity of linking equity compensation to socially responsible practices
Social responsibility is an increasing priority for corporates, reflecting changing pressures from stakeholders and society. In this article CGLytics looks at the trend of linking executive equity compensation to responsible social practices.
The Effect of Executive Departures on Company Performance
The Executive Management Team plays a pivotal role in the performance of a company. The dismissal or exit of one or more executives is often accompanied by a change in strategy. However, this isn’t always perceived as a positive change by investors.
2019 CEO Pay Review: The Top 50 Highest Paid CEOs
As proxy season progresses and companies file their annual reports, CGLytics surveys the world’s highest paid CEOs (so far) and looks at how executive compensation has grown since the last year.
Data puts CEO rewards into perspective in the Netherlands
In this article, originally published in Dutch in Mgmt. Scope, CGLytics examines CEO pay in the Netherlands and how it is one of the hottest topics in shareholder discussions.
In the spotlight: EQT Corporation Proxy Statement
In this article, CGLytics takes a look at the upcoming EQT Corporation AGM resolutions and how the CGLytics platform analytics can help promote engagement between the company and shareholders.
Growing Expectations of Director Responsibilities and Evolving Attitudes Towards Overboarding
CGLytics takes a look at how the role of the board is changing, and how directors are having to rapidly become experts in a range of topics in which they have little to no previous experience.
The Top 50 Highest Paid CEOs
As proxy season progresses and companies file their annual reports, CGLytics surveys the world’s highest paid CEOs (so far) and looks at how executive compensation has grown since the last year.
The EU Shareholder Rights Directive: The implications for executive compensation in Belgium and Luxembourg
The corporate governance landscape is changing. Listed EU companies are increasingly subject to more disclosure and transparency requirements while executive compensation is now under greater scrutiny than ever. In this article CGLytics takes a look at the implications of the EU Shareholder Rights Directive on executive compensation in Belgium and Luxembourg
Gender Diversity In Australia
CGLytics explores boardroom gender diversity in Australia, with a particular focus on the upcoming ASX diversity targets.
Waivers Of Mandatory Retirement Ages And Company Performance
CGLytics examines the costs and benefits of waiving the mandatory retirement age for directors, elaborating upon aspects of the S&P 500: Increasing Boardroom Diversity Report.
Dominant themes from the 2018 AEX proxy season and what to expect in 2019
During the 2018 Dutch proxy season, shareholders actively engaged in a range of governance matters, with CEO remuneration remaining a key focus for stakeholders and widely publicised by the media. And, as always, the upcoming 2019 proxy season is likely to be influenced by happenings from the previous year.
AI in the Boardroom: Fantasy or Reality?
While the exact role of AI in the boardroom in up for debate, the question remains: has the Robo Director come of age?
Younger directors on S&P 500 boards show positive effect on companies’ performance
Bringing younger directors into the boardroom does not only add value in terms of unique perspectives and improved innovation, but also impacts company performance
S&P 500 companies are listening: One third of new board appointments in 2018 were women
Gender diversification on boards was a prominent issue in 2018 and shareholders had never been more explicit in their expectations of companies.
Action needed despite third of new S&P 500 board appointments being women
Gender diversification on boards was a prominent issue last year, and shareholders have never been more explicit in their expectations of companies, writes Aniel Mahabier
What’s New for the 2019 Proxy Season?
By looking at the UK, which currently has the spotlight on corporate governance practices, we can be sure that company boards will be compelled to implement good governance practices.
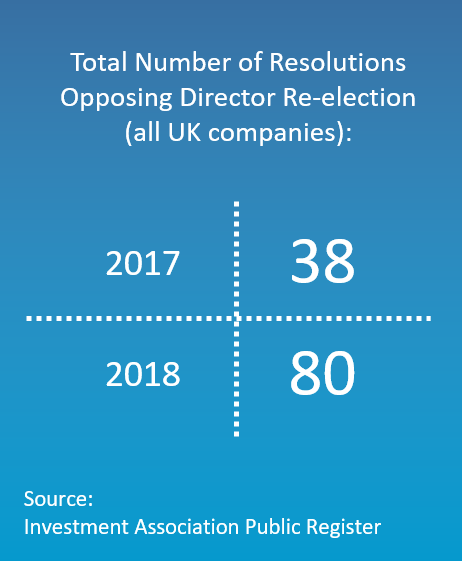
Be prepared: Learnings from the UK 2018 proxy season
The UK is at the forefront of shareholder concern of good corporate governance practices. In a climate of increasing proactive shareholder engagement, the CGLytics FTSE 100 2018 proxy season review evaluates underlying trends to provide unique insights for being prepared for the forthcoming season.
What is ESG?
What is ESG? ESG (Environmental, social and governance) criteria are of increasing interest to companies, their investors and other stakeholders. With growing concern about he ethical status of quoted companies, these standards are the central factors that measure the ethical impact and sustainability of investment in a company. ESG factors cover a wide spectrum of … Continue reading "What is ESG?"
What is Corporate Governance?
Corporate governance is the system of rules, procedures and processes by which a company is controlled and directed. In practice, governance is concerned with balancing the combined interests of a company’s stakeholders, including shareholders, management, staff, customers, suppliers and the community in which it operates.
CGLytics is a leading Corporate Governance Analytics provider, delivering unique insights, real-time data and benchmarking tools, in a single convenient software solution.
With Over 1 Billion Data Points and Powerful Algorithms, CGLytics is Utilised by Leading Investors and Proxy Advisors.
5,500+
Listed Companies

125,000+
C-Level Profiles

8 MIL+
Company Disclosures
and Filings

800 Mil+
N-PX proxy voting
resolutions

1.3 Mil+
Relationships Connections

Key financial metrics from 2008 onwards for predictive analysis and companies top 25 ownership data

More than ten years of historical compensation data and array of unique performance indicators

Current and historical board composition, skills data and millions of business relations

Curated governance news in real-time